Fist law of thermodynamics
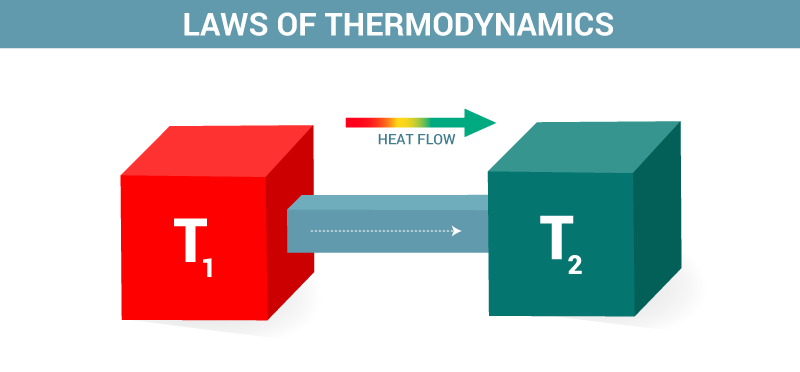
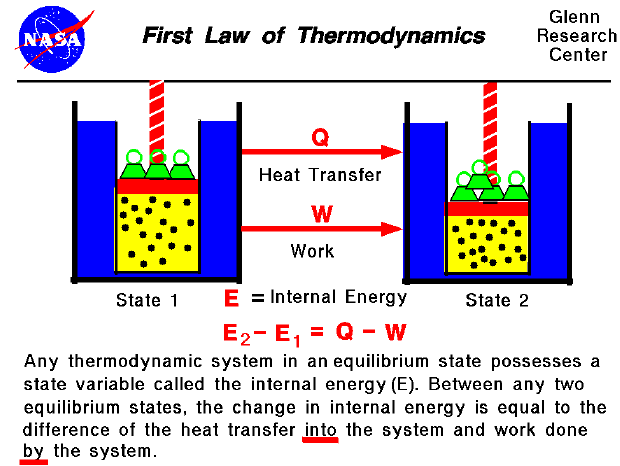

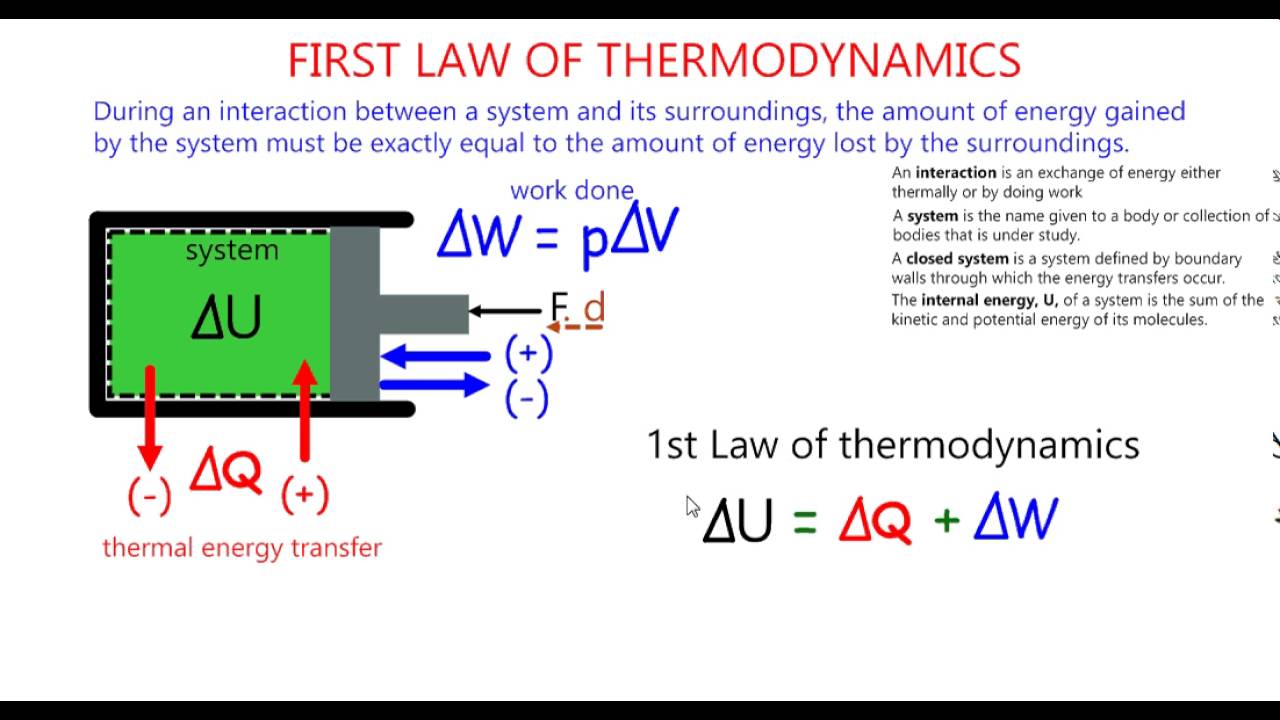
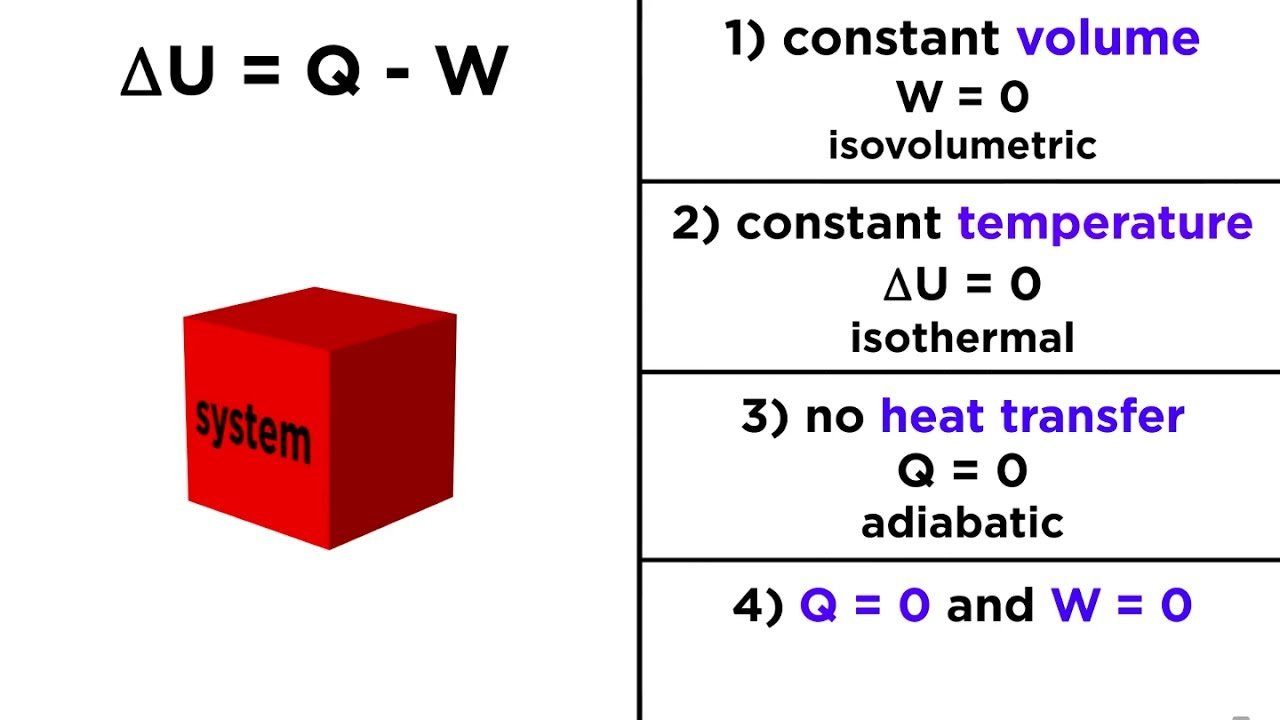

The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic systems. The law of conservation of energy states.
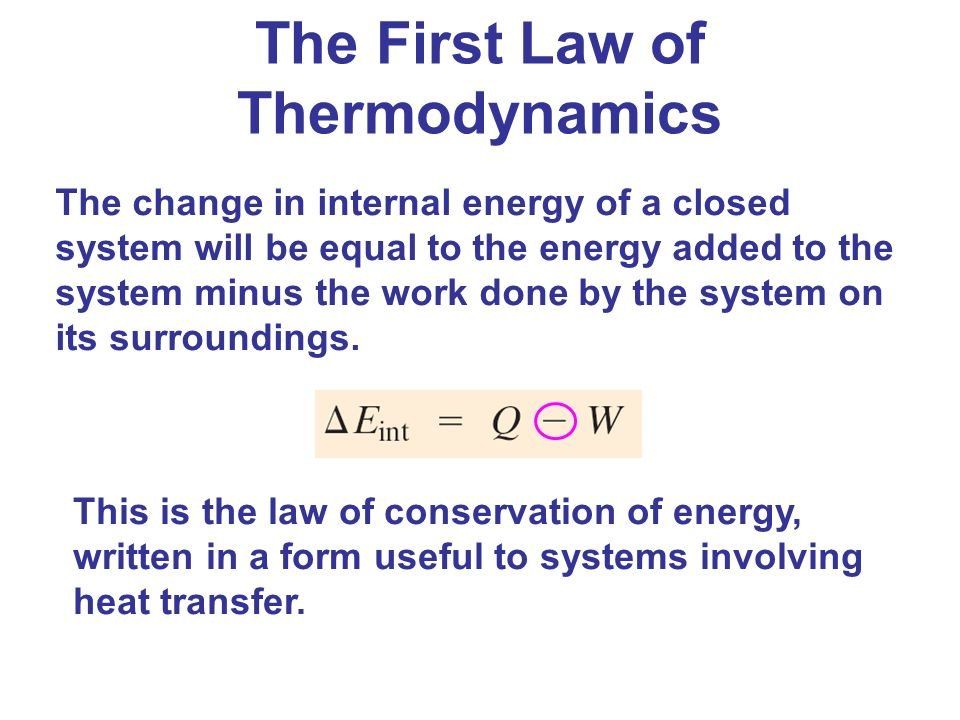
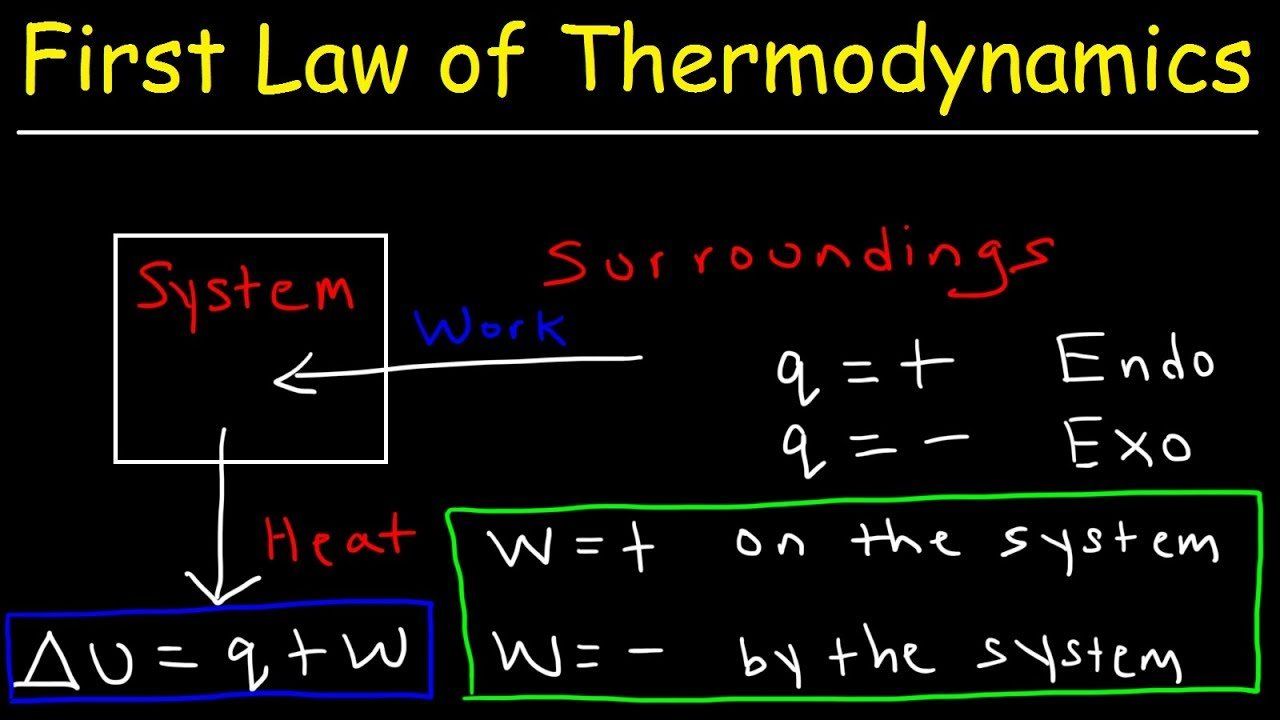
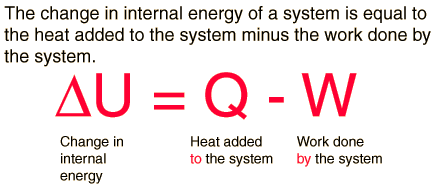
The first law of thermodynamics is the application of the conservation of The first law makes use of the key concepts of internal energy, heat, and system work.

6 people find in your city who like Swapping. Look

Keep Exploring Britannica
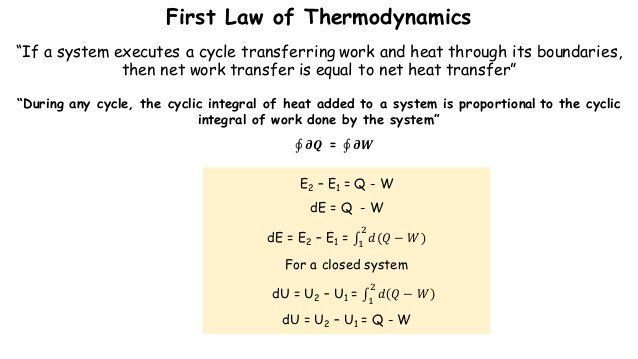


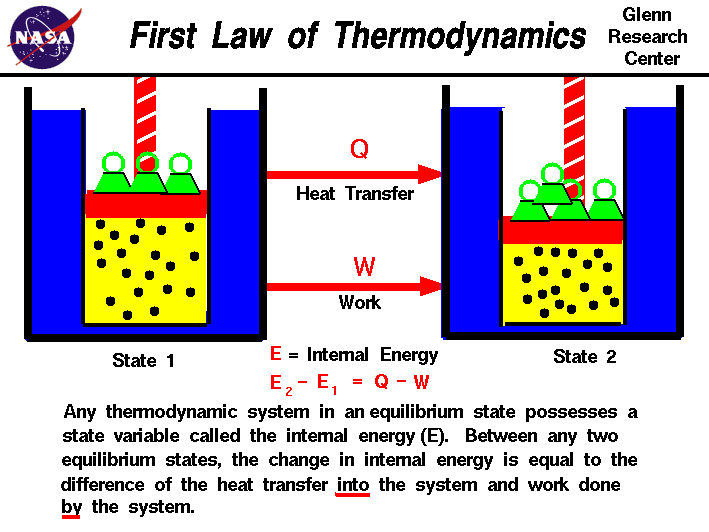
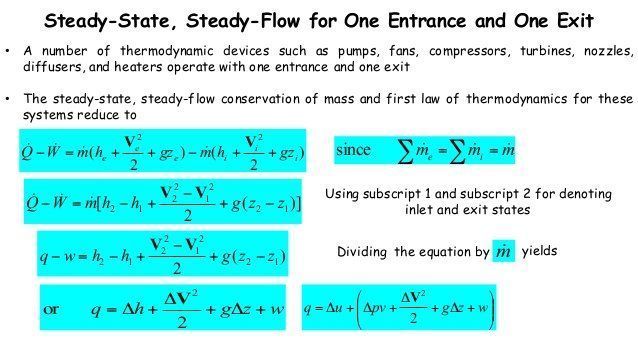
The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring.


Philomena Age: 22. I'm available for serious requires in France, currently MonacoCoco ChanelHi,guys and thanks for taking a moment to stop at my profileSensual greetings,
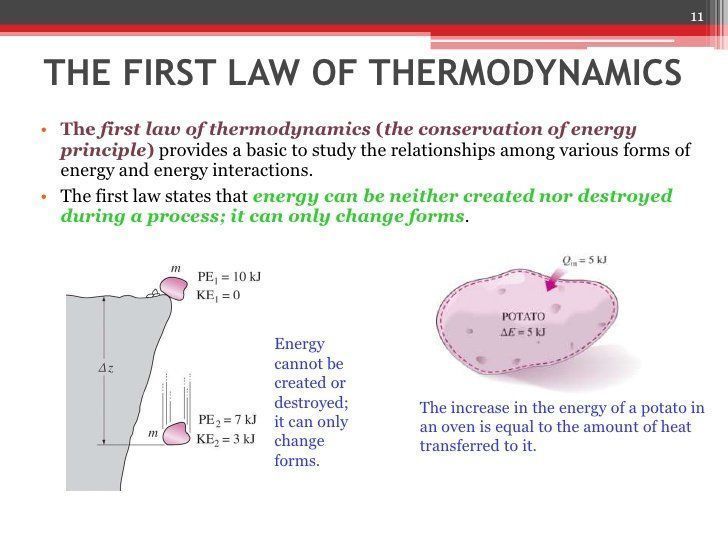
Description:Contributors To understand and perform any sort of thermodynamic calculation, we must first understand the fundamental laws and concepts of thermodynamics. For example, work and heat are interrelated concepts. Both work and heat together allows systems to exchange energy. Energy cannot be created nor destroyed, but it can be converted or transferred. With the interactions of heat, work and internal energy, there are energy transfers and conversions every time a change is made upon a system.









































User Comments 4
Post a comment
Comment: